Coupling Endergonic and Exergonic Reactions
The sum of the ΔG values of the two reactions is then negative. ATP then diffuses to another place in the cell where its hydrolysis releases the free energy to drive an endergonic reaction.
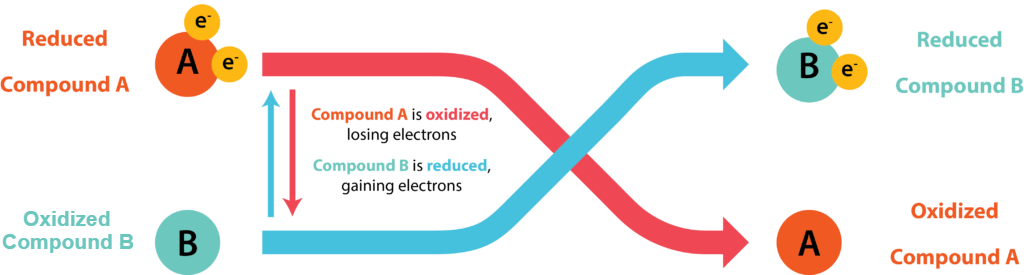
Energetics Redox Reactions General Microbiology
Importance of Energy Coupling.

. All of the choices are correct. Lost as nonusable heat to the environment. The sum of the ΔG values of the two reactions is then negative.
The reaction ADP P -- ATP is a n _____ reaction. The specific chemical reactions are called. Exergonic and endergonic reactions.
The hydrolysis process of any ATP molecule facilitates in the breakdown of high-energy bonds phosphate bonds. Energy coupling of endergonic and exergonic reactions within cells a. Exergonic and endergonic reactions are kind of glossed over in most chemistry classes.
In the process high measures of energy are released in an exergonic form. The sum of the ΔG values of the two reactions is then negative. An exergonic reaction is one in which ΔG increases and an endergonic process is one in which ΔG decreases.
The process is called energy coupling. Utilizes ATP to carry energy between the d. In this lab you will create an endothermic and an exothermic reaction.
Chemical Transport Mechanical To do work cells manage energy resources by energy coupling the use of an exergonic process to drive an endergonic one Most energy coupling in cells is mediated by ATP. The process is called energy coupling. The only way that an endergonic reaction can occur spontaneously is if it is coupled with an even more exergonic reaction.
The overall reaction becomes exergonic and spontaneous. The key to coupling exergonic and endergonic reactions is the formation of this phosphorylated intermediate which is more reactive less stable than the original unphosphorylated molecule. Used to drive an endergonic reaction.
Coupling occurs when the energy released by an exergonic reaction is. The ATP molecule is the key to energy coupling. Do endergonic reactions have to be coupled with exergonic reactions.
Uses heat released by one reaction to fuel the other reaction. Energy coupling is the cells ability to use the energy released from exergonic reactions is to drive endergonic reactions. An endergonic reaction occurs by coupling with an even more exergonic reaction.
Chemical work --pushing of endergonic reactions not. Second Law of Thermodynamics and entropy. The cellular processes of energy intake and output are called endergonic or exergonic.
The coupling process helps to convert the energy generated into an endergonic form ensuring that the energy is not lost as heat. Coupling of exergonic and endergonic reactions is very common in metabolism. You have to pick the.
Google Classroom Facebook Twitter. Permits biological reactions to proceed at temperatures consistent with life. Its easy to see why because they can be very confusing.
Energy coupling Combustion is an example of an exergonic reaction. Do endergonic reactions have to be coupled with exergonic reactions. Solution for Coupling Endergonic Exergonic Reactions in Metabolism Hesss Law.
The process is called energy coupling. Use the appropriate AG values below from the table. ATP structure ATP hydrolysis to ADP and reaction coupling.
Do endergonic reactions have to be coupled with exergonic reactions. First Law of Thermodynamics introduction. The process of using the products of an exergonic reaction to carry out an endergonic reaction is called.
Free energy is captured and retained in the PO bonds of ATP. In cells what is usually the immediate source of energy for an endergonic reaction. The overall reaction becomes exergonic and spontaneous.
The laws of thermodynamics. Coupling exergonic reactions to endergonic reactions A cell does three main kinds of work. An endergonic reaction occurs by coupling with an even more exergonic reaction.
All of the choices are correct. An exergonic reaction is one in which ΔG increases and an endergonic process is one in which ΔG decreases. What is the fate of the phosphate group that is removed when ATP is converted to ADP.
Reaction coupling to create glucose-6-phosphate. The only way that an endergonic reaction can occur spontaneously is if it is coupled with an even more exergonic reaction. Yes combustion is an.
The energy for an endergonic reaction comes from a n _____ reaction. Used to decrease the entropy of the universe. The only way that an endergonic reaction can occur spontaneously is if it is coupled with an even more exergonic reaction.
The only way that an endergonic reaction can occur spontaneously is if it is coupled with an even more exergonic reaction. Used to drive another exergonic reaction. What is the key to coupling exergonic and endergonic reactions.
The only way that an endergonic reaction can occur spontaneously is if it is coupled with an even more exergonic reaction. Energy coupling of endergonic and exergonic reactions within cells permits biological reactions to proceed at temperatures consistent with life uses heat released by one reaction to fuel the other reaction and utilizes ATP to carry energy between the exergonic and endergonic reactions. The overall reaction becomes exergonic and spontaneous.
The recipient with the phosphate group covalently bonded to it is then called a phosphorylated intermediate.
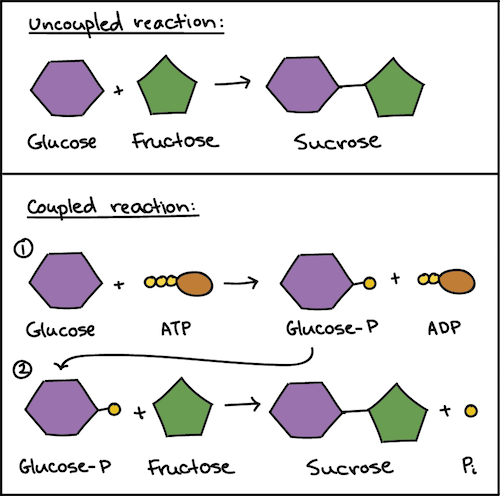
Atp Cycle And Reaction Coupling Energy Article Khan Academy
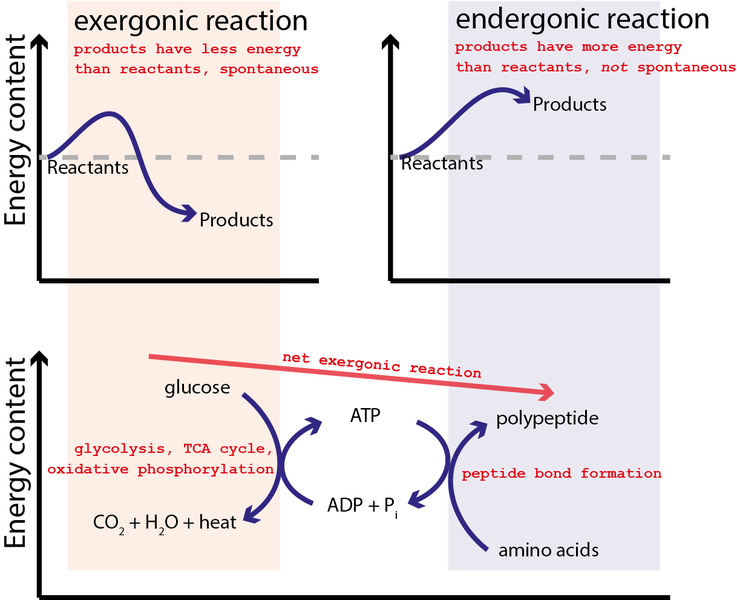
9 6 Coupled Reactions Chemistry Libretexts

Endergonic And Exergonic Reactions Feedback Inhibition Youtube


0 Response to "Coupling Endergonic and Exergonic Reactions"
Post a Comment